Crystal structures of the seven-transmembrane domain of human GLP-1R determinedmodulation in class B
A team of scientists in Shanghai has recently determined crystal structures of the human glucagon-like peptide-1 receptor (GLP-1R) and revealed molecular mechanisms of allosteric modulation in class B G protein-coupled receptors (GPCRs). The results, described in a paper entitled “Human GLP-1 receptor transmembrane domain structure in complex with allosteric modulators”, were published online on May 17, 2017 (18:00PM, London time) in Nature (http://www.nature.com/nature/journal/vaop/ncurrent/full/nature22378.html).
Secreted by the L cell in the gut, glucagon-like peptide-1 (GLP-1) exerts its glucose control action through GLP-1R expressed on the surfaces of many cells (e.g., pancreatic β cells). GLP-1R is a well-recognized drug target for type 2 diabetes, exemplified by several peptidic therapeutic agents on the market, with combined annual sales of billions of dollars.
Crystallographers have long been frustrated by low yields and instability of GLP-1R protein preparations. It was successfully solved by making a double fusion protein construct combined with rational mutations based on a structural model. The fusion protein was finally crystallized to 2.7 angstrom in complex with two negative allosteric modulators (NAMs), respectively. Both structures are in an inactive conformation with each NAM bound in a similar pocket outside of helix VI. However, their binding modes are distinct as confirmed by the extensive mutagenesis studies. Molecular modeling and mutation analysis suggest that agonist positive allosteric modulators (agoPAMs) target the same general region, but in a distinct sub-pocket. While the NAMs block the activation of GLP-1R by inserting into the cavity between helices VI and VII, agoPAM binds mainly to the space between helices V and VI, thereby allowing the outward shift of helix VI, a phenomenon associated with G protein coupling in class A GPCRs.
Since most peptide drugs are administrated via non-oral routes, orally available GLP-1 or its small molecule surrogates have been vigorously sought. Subsequent to the discovery of Boc5, the first non-peptidic GLP-1R agonist, by Dr. Ming-Wei Wang’s team in 2007, no significant progress had been made. Determination of the three-dimensional structure of GLP-1R is thus helpful to better design and develops new therapeutics targeting the receptor.
The team efforts were led by three senior authors of this paper, Drs. Raymond Stevens, Ming-Wei Wang and Zhi-Jie Liu. Drs. Gaojie Song and Dehua Yang played key roles in the experiments. Other contributors to this work include local scientists as well as those from the Netherlands, Denmark and United States. National Natural Science Foundation of China, National Health and Family Planning Commission, Ministry of Science and Technology of China, Chinese Academy of Sciences, Shanghai Science and Technology Development Fund, and GPCR consortium supported the study.
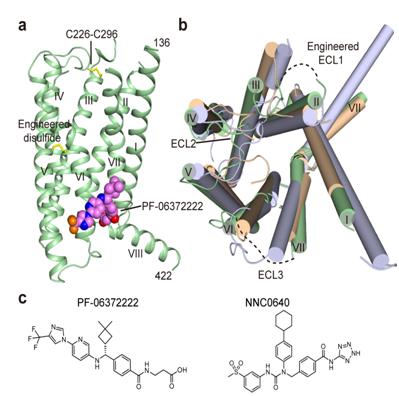
Crystal structure of the transmembrane domain of GLP-1R.



Click here download: GLP-1R.pdf
